ADF STAFF
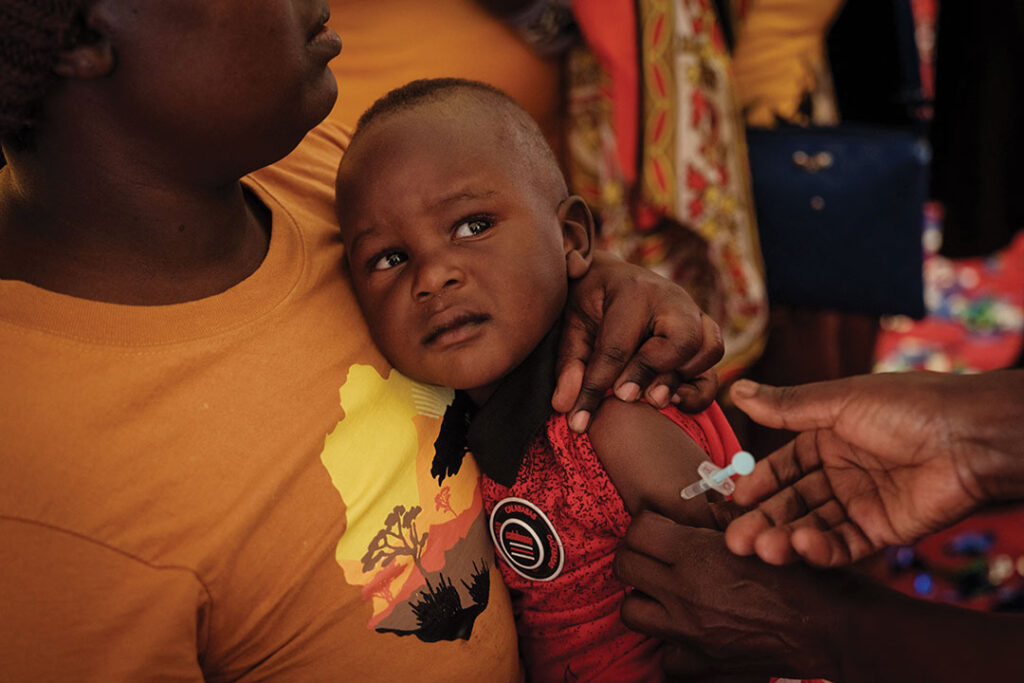
The World Health Organization (WHO) in October 2023 approved a second malaria vaccine two years after it approved the first one. The move is expected to ease concerns about availability in Africa.
The R21/Matrix-M vaccine, developed by Britain’s Oxford University, will join the RTS,S/AS01 vaccine in preventing the mosquito-borne disease in children. Malaria kills hundreds of thousands of children each year.
“The addition of R21 to the list of approved shots is expected to result in sufficient supply to benefit all children living in areas where malaria is a public health risk,” the United Nations reported soon after the WHO decision.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria,” said WHO Director-General Dr. Tedros Adhanom Ghebreyesus of Ethiopia. “Now we have two.”
Dr. Matshidiso Moeti, WHO regional director for Africa, said the shot “holds real potential to close the huge demand-and-supply gap. Delivered to scale and rolled out widely, the two vaccines can help bolster malaria prevention and control efforts and save hundreds of thousands of young lives in Africa from this deadly disease.”
Another advantage of the new vaccine is its low cost, which is between $2 and $4 per shot.
The vaccine was set to debut in some African countries, including Burkina Faso, Ghana and Nigeria, in early 2024. It was expected to be available in other countries by the middle of the year, Tedros said.